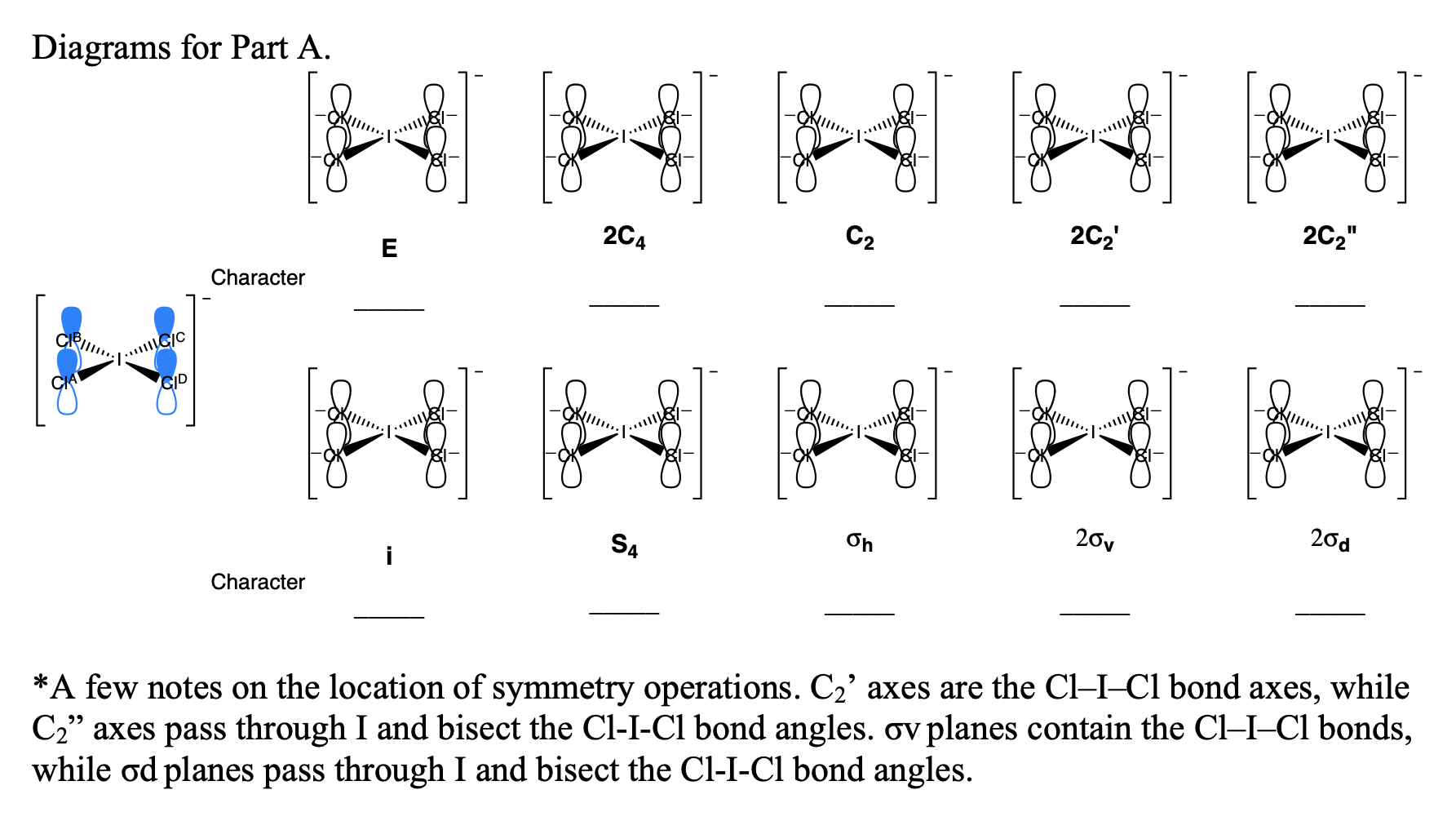
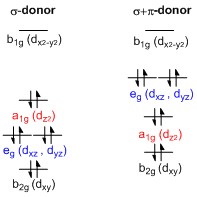
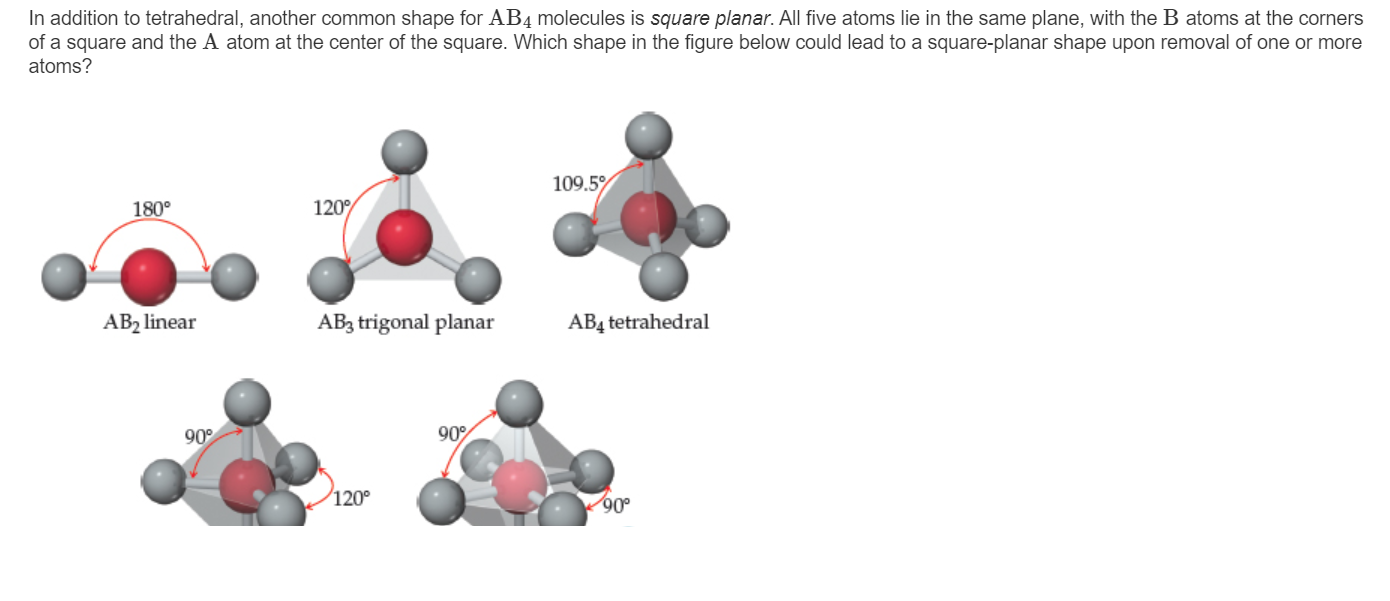
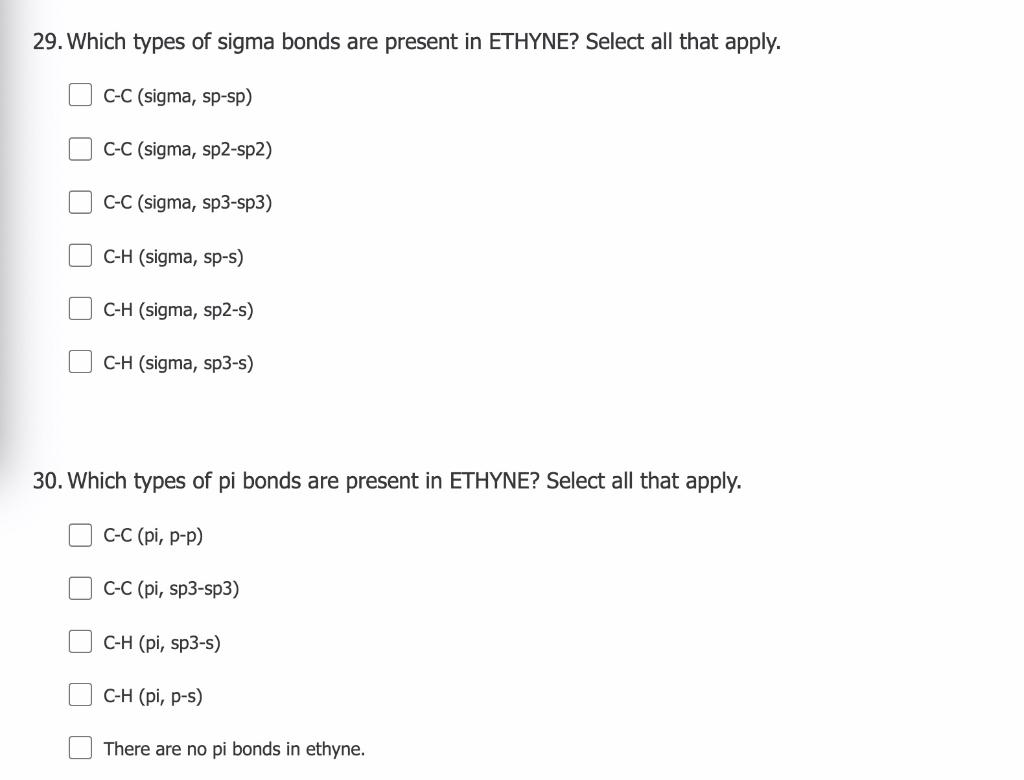
What makes a seesaw-shaped molecule instead of a square planar or tetrahedral? I understand that tetrahedron has 0 lone pairs, but what about the others? - Quora

Tetrahedral (left) and square pyramidal (right) distortions for square... | Download Scientific Diagram
What makes a seesaw-shaped molecule instead of a square planar or tetrahedral? I understand that tetrahedron has 0 lone pairs, but what about the others? - Quora
What makes a seesaw-shaped molecule instead of a square planar or tetrahedral? I understand that tetrahedron has 0 lone pairs, but what about the others? - Quora

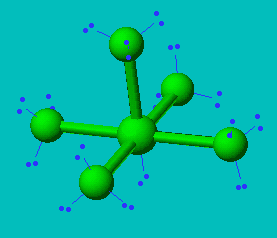
Seesaw Molecular Geometry - Seesaw Shaped Molecules, Lone pairs, Examples & Hybridisation of Seesaw Molecular Geometry
Stereochemistry Square Planar Molecular Geometry Point Line Atom, PNG, 575x534px, Stereochemistry, Area, Atom, C Standard Library,
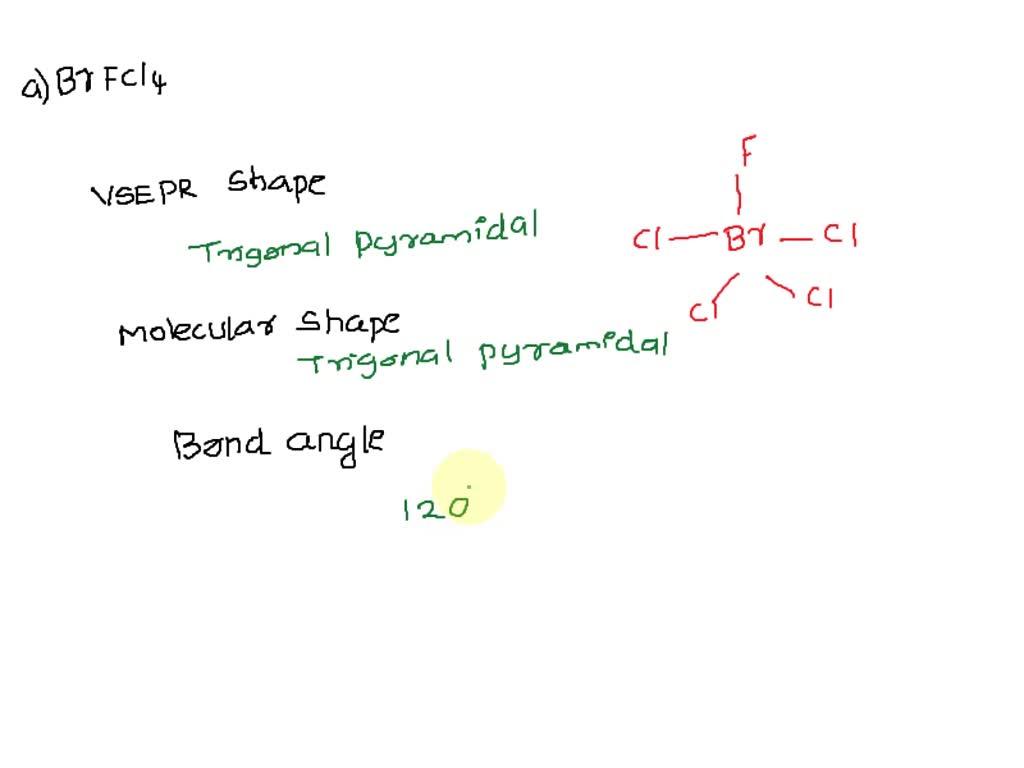
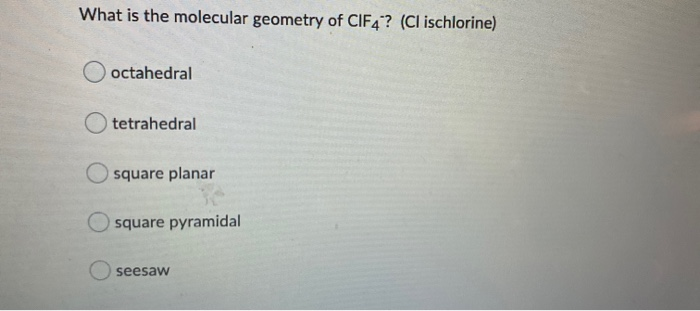
SOLVED: For each of the following, choose the VSEPR shape, molecular shape and minimum approximate bond angle from the drop-down menus to complete the sentences. (a) The cental atom of BrFCl4 has
What makes a seesaw-shaped molecule instead of a square planar or tetrahedral? I understand that tetrahedron has 0 lone pairs, but what about the others? - Quora
![16-Square planar [4]-fold geometry and its off-plan distortion along... | Download Scientific Diagram 16-Square planar [4]-fold geometry and its off-plan distortion along... | Download Scientific Diagram](https://www.researchgate.net/publication/313641543/figure/fig17/AS:669448675545111@1536620410383/Square-planar-4-fold-geometry-and-its-off-plan-distortion-along-the-C-4-axis.png)
16-Square planar [4]-fold geometry and its off-plan distortion along... | Download Scientific Diagram
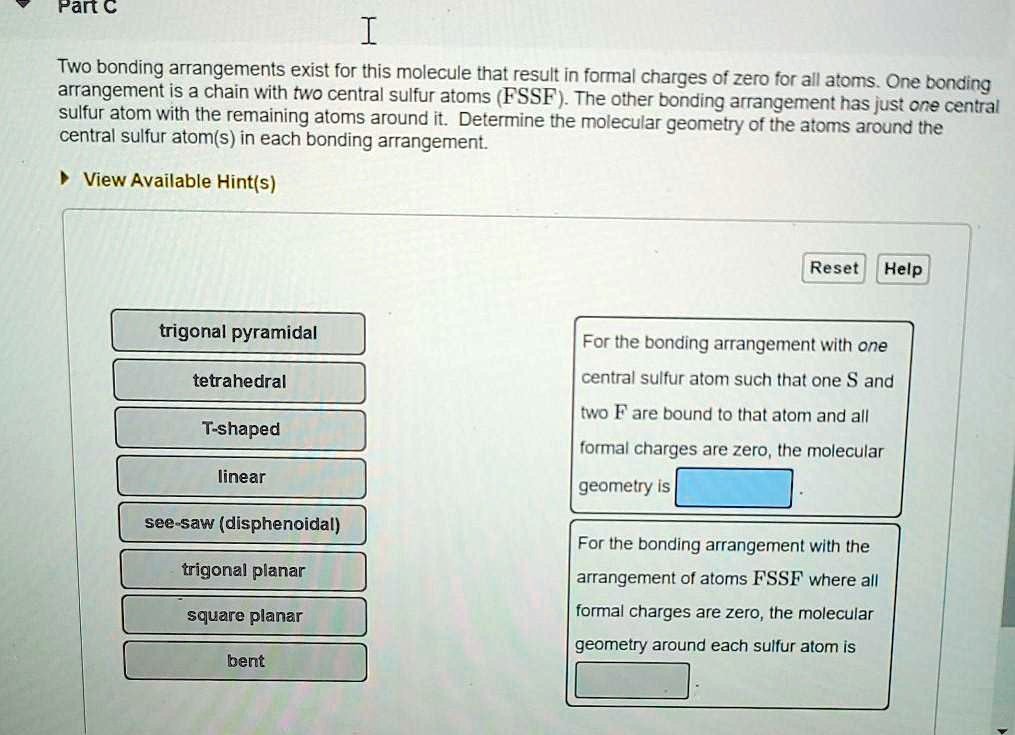
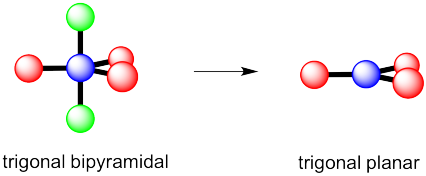
SOLVED: Pan € arranrerg arangernents exist for this molecule thalresyit In formal charges 0f zero for all atoms. One bonding arrangement is 3 chain with two central sulfur atoms (FSSF . The